CHAPTER III: HOW PLANTS PRODUCE THEIR YOUNG
THERE is perhaps no device of nature that more perfectly accomplishes its purpose than the one with which all living things are endowed—the instinct for the renewal of life. In man the dawn of the mating instinct has ever been the theme of poets, and some of its manifestations are the despair of ascetics. Through it some of the noblest of man’s emotions have arisen, and because of its perversion our daily newspapers chronicle the basest and most sordid tragedies.
But whether noble or ignoble, this instinct for mating is, in its simplest terms, only a provision of nature that all life contains within itself the means of renewing life. Without this, life, so far as we know it, would end with the present generation. Perhaps our understanding of this decree of an all-wise nature to increase and multiply will be heightened by looking at it not only from its familiar manifestations in man, but more broadly. Seen from this broader viewpoint, it is the inherent legacy of all living things from the dawn of life on the earth down to the present. Even the simplest one-celled organisms have the faculty of increasing. In all plants, both the flowerless ones and those producing flowers, the process is carried to a perfection almost unbelievable in its intricacy and in provisions against its failure. From the matings of flowers much may be gleaned; even man himself can learn from them the capacity for sacrifice, the sinking of individual aims and pleasures in the greater scheme of conforming to that necessity for renewal of the race upon which all progress must be based.
The equipment which different flowers have developed for this purpose, their almost uncanny devices to make certain that only the distant and foreign male can ever impregnate the female, the enormous wastage of both unfertilized females and males that will never become fathers, and the overwhelming effectiveness of it all, in spite of this prodigality—these manifestations of the production of young in the plant world will take up the rest of this chapter. All the first part will tell of this process in flowering plants, while the second shows how flowerless plants accomplish the same end in more secret ways. Finally, in a brief third part, we shall see how, without mating of the sexes, nature has still one other way to see to it that there is a constant supply of young.
We have already made clear that all plants are divided upon the basis of whether they bear flowers and their mating goes on before the world, or whether they bear none and the process is accomplished in more secret ways. Because flowers are so much better known, and it is simpler to see how the act is consummated in them than in the cryptogamous plants, we shall first consider the phanerogams or flowering plants, and in the second section of this chapter the cryptogams or flowerless plants.
1. Visible Marriage of Flowering Plants
In the first chapter, under the section devoted to flowers, we found that the stamens are the male and the pistils the female organs of reproduction. As the period for mating draws near there is developed in the anther, which is the enlarged tip of the stamen, a fine, usually yellow, powder known as pollen. This matures in the anther, and when ripe is discharged from tiny pores.
Pollen is made up of individual pollen grains, which are very often stuck together so that we see only the mass, not the individual pollen grain. Sometimes the pollen is not sticky, as in the case of pine trees or in the ragweed—a fertile cause of hay fever. In these, and hundreds of other plants, the wind will blow great clouds of pollen through the air. When we stop to consider that a single, or at most a very few pollen grains are all that are necessary—in fact, are all that can be of real service—the enormous wastage of the male fertilizing substance, in order that mating be secured, gives us some idea of how prodigal is nature in this supreme function.
The pistil, or female organ of reproduction, is more cautious in the expenditure of its resources. As we have seen, it is composed of a swollen base, the ovary, a slender shank, the style, and a swollen or branched tip, the stigma. In some plants the ovary is divided into several compartments or cells, each with one or more ovules, which are only immature or unfertilized seeds, often very tiny, but usually quite easily seen if the ovary is cut open. It is the entrance of the pollen grain into this ovule that consummates the act of fertilization. As the ovule is carefully secreted within the ovary of the flower, and as the male fertilizing stuff or pollen is found only on the anther, it is obvious that some method of bringing the two together must be provided for.
In some plants this is accomplished by the anthers being just above the stigma, and when the pollen is ripe and the ovule ready, the stigma is found to be covered with a sticky substance. As the falling pollen grains touch the stigma, they are caught in this sticky substance just as surely as flies are caught once they touch a fly paper. But just here one of the most wonderful processes of nature begins. The pollen grain begins, slowly at first, to grow, and in the act it penetrates the outer coat of the stigma with a minute pollen tube. This slender threadlike tube, carrying with it the male germ, grows straight down through the stigma, into the narrowed style, and through this to the ovule. Once the pollen is caught on the stigma, nothing is so sure of fulfillment as that this male fertilizing stuff will ultimately reach the ovule. For the hitherto virgin ovule this impregnation starts a new phase in life. It means the beginning of the end, but in the process fruit and seed will be developed, and the young bride, already a mother, has triumphantly accomplished that for which she exists.
If fertilization of all flowers were as simple as this, there would be no need of what follows, but actually in surprisingly few plants are the stamens and pistils so arranged, the ripening of the pollen and readiness of the ovule for impregnation so timed that the act can be accomplished in such direct fashion. For it is quite obvious that in flowers in which the whole drama of mating goes on within the petals, without the interference or help of any outside agency, the result will be a crop of young who know no other characters than those of the parents, and have nothing to look forward to but a closely inbreeding progeny, very little, if at all different from themselves. In other words, such plants are pure bred, they lack the usually obvious virility that comes from crossing the male of one plant with the female of another. There are so many devices to prevent self-fertilization in flowers, so marvelous are the contrivances to see to it that only cross-fertilization can be effective, and, finally, the experience of breeders that strength and virility often or usually result from impregnating the ovules of one plant with the pollen of another, that we are forced to the conclusion that absolute purity in the sexual relations of flowers is rare indeed. It occurs, without peradventure of a doubt, only in those flowers whose petals never open and where fertilization is consummated, if not in private, at any rate without external help. In many violets the showy violet blossoms are often nearly infertile, while down near the ground are inconspicuous flowers which never open, but within which fertilization is so successful that the crop of seeds is far more plentiful than in the more showy ones that most people think are the only flowers ever borne by violets. These flowers that never open, or at any rate open so slightly that their sexual processes are modestly completed without intrusion, are known as cleistogamous flowers (Figure 69). They have been found in a few plants, but overwhelmingly the greater number of flowers not only do, but must, rely on some outside agency to insure fertilization.
Certain structural features of flowers have been so developed that fertilization of the ovary by the pollen of the same flower is impossible. The commonest case is in those flowers where the stamens are shorter than the pistils, as they always are in the common snowdrop, hyacinth, the sassafras tree, and in hundreds of others. There can be no consummation of the reproductive process in such flowers without some outside aid. More futile still without this aid are those flowers where the stamens are well above the pistils, but the time of maturing in both differs by a few days or even hours. Nothing could be more helpless than the pistil under these circumstances, for if its instinct for maternity were ever so strong, it would be doomed to barren sterility by the premature development of the males. Sometimes, too, the female is prematurely ripe for impregnation, and the stamens lag behind a day or two. Her time passes and with it her only chance of fertilization—by her own haste she has rendered impotent the now useless pollen which appears doomed to fall aimlessly upon the unreceptive stigma.
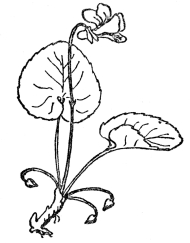 FIG. 69—THE VIOLET Note the showy, often partly infertile upper flowers and the much smaller cleistogamous ones at the base, which never open and yet produce a good crop of seeds.
FIG. 69—THE VIOLET Note the showy, often partly infertile upper flowers and the much smaller cleistogamous ones at the base, which never open and yet produce a good crop of seeds.
But, perhaps, the most hopeless of all is the well-known partridge berry, whose red berries are common in the woods during August and September. This seems as though it fought off any chance of securing a mate by a flower structure and behavior that would certainly so result if some way out of the difficulty were not at hand. The partridge berry bears two kinds of flowers that outwardly look much alike, but whose sexual organs differ in this way: in some flowers the stamens are all shorter than the pistil, and in others the pistil is much outtopped by the stamens. The extraordinary feature of it is not so much this structural difference, however, but the fact that pollen from the short-stamened flower is useful only to its neighboring short-styled relative, while the pollen from this long-stamened but short-styled neighbor is nearly useless where it is found and really useful only on the long-styled plant. By this device, but again only with outside aid, this plant does not prevent maternity, but increases its chances of being fruitful, for, as we have already seen, cross-fertilization appears to be the rule rather than the exception, and the partridge berry not only needs it, but can exist only when its offspring are the result of such crosses.
In all those plants that bear the different sexes in different flowers on the same plant, as in the hickory, or even on different plants, as in the willow, there must, of course, be some method arranged for cross-fertilization or they would promptly die out. So general is this cross-fertilization, so much a part of the economy of nature does it appear to be, that we can only think that there must be in the production of this vast horde of the cross-fertilized some advantage. Besides securing greater virility, which almost certainly results from this promiscuity, greater variability is promoted. If virility is the result, the price paid for it is tremendous, for the hindrances to self-fertilization are so many and so effective that most flowers would inevitably die as perfectly pure but ineffectual virgins if that fatality were not prevented. How they are saved from such a sterile fate, how they finally secure a mate by devices that outshine the most bewitching tricks of the daughters of Eve, is one of the most fascinating stories in all the history of the plant world.
For, of course, flowers do secure a mate, and they are aided in this enterprise by the most formidable array of helpers, one might almost call them conspirators. The chief of these are insects, thousands of different kinds of which are constant flower visitors. Some of the smaller birds, and even snails, also help flowers to meet their mates. The wind, too, bears pollen through the air to some expectant bride-to-be. And, finally, in the water, by a series of acts the like of which no one could improve for cunning, the cross-fertilization of certain aquatic plants is consummated. It would take a book larger than the present one to give even the briefest account of how these different aids to maternity do their work and how the flower responds to this help. As that is quite out of the question, only some of the best-known examples of cross-fertilization will be given, and these will be grouped according to what agency the flower is indebted for its chance of doing that for which it is created.
INSECTS AS FLOWER VISITORS
On any summer day, especially when the sun is shining brightly, we may see bees and butterflies flitting from flower to flower, busy as the proverbial bee. We already know enough about nature’s ways of doing things to be certain that these, and hundreds of other kinds of insects, do not come for nothing, and that the flower must have something to offer. Bees, especially, are thrifty creatures whose business demands exacting and prolonged toil. They would not waste five seconds upon idle flower calling if the blossoms did not yield a rich store. And thousands of flowers do yield the sweetest and richest kinds of stores of nectar or honey, which is, in fact, by the help of insects who alone can extract it, our sole source of honey. Many flowers which produce no nectar do have such plentiful stocks of pollen that the bees come for that alone. In the peony, for instance, over three million pollen grains are produced in each flower, only a minute fraction of which can ever fertilize an ovule. All the rest would be wasted were not pollen in itself a particularly nutritious food for young bees, and consequently much sought after by the careful bee mothers. They are the only insects that feed their young on pollen, or beebread, as it is called by the beekeepers, so that the enormous overproduction of this male fertilizing agent, from the point of view of the flower, is a decided attraction, one might almost call it a trap, to insure constant visitations from bees. For they are perhaps the most useful of all insects in the great game of securing cross-fertilization, as we shall presently see. Many other kinds of adult insects eat pollen directly and so add to the number of insect visitors.
No one has ever been able to explain the beautiful coloring of flowers, except that it serves as an attraction for insects and small birds. Like the honey or nectar, it seems to play no real part in the home economy of the flower, to be of not the least use otherwise. While honey and the gorgeous colors of flowers are a delight to man, that would be no sufficient reason for the ability to produce them. Both of these attributes of flowers, as attractions for insect visitors, are, however, so absolutely essential to cross-fertilization that we must think of them as having grown up out of that demand. As we shall see a little later, even the structure of some insects has been modified so that they can reach the nectar or pollen only by automatically doing for the flower what it cannot do itself.
While color of flowers seems as though it were attraction enough, it is very likely that their fragrance or perfume is still more seductive in its power of luring insect visitors and repelling useless ones. Poets have called this perfume the soul of the flower, and in its almost intangible beauty it might well be so called were it not for the fact that it appears to be of not the slightest use, except as a lure. In all the equipment of seduction there is none like this fragrance of flowers for attracting insects.
Flowers, then, have things to offer to insects which the latter need. Nectar and pollen are the chief, and where these merely bread and butter objects are not enough, or sometimes in addition to them, the flower is dressed out in gorgeous colors or perfumed with a fragrance beyond the dreams of the fairest bride. What insects do to complete the fertilization of such a legion of beauties makes up the romance of the flowers. Perhaps not even in man himself is this creation of new life so surrounded with beautiful ideas. Also, as in man, it sometimes is bound up with an almost fiendish cruelty and cunning. Some of these visitors and what they do, but unfortunately only a very few, can be mentioned here. They must serve as types or examples of what is going on all about us on any summer day.
The common blue columbine, much grown in gardens for its beautiful blossoms, always has the flowers hanging upside down, a habit that admirably serves to keep its pollen from rain. The opening and closing of many flowers in cloudy weather, or at night, may be for the same reason. Everyone knows the five blue spurs into which the petals of columbine are produced. At the very end of each spur, which is always curved, the flower secretes a considerable quantity of honey. This, one of the greatest attractions to bees, leads inevitably to a visit from one. The bee, in order to reach the honey, hangs on to the inverted flowers, clutching the base of the spur with its foreleg, and further securing itself by the mid or hind legs, which grasp the slender column into which, in the columbine, the stamens and pistils are crowded. In its anxiety to reach the honey the bee pokes its head as far into the spur as possible, but it gets in only a fraction of the full length of the tube. To reach the honey it extends its sucking apparatus, which is a complicated mechanism for this purpose on the head of nearly all insects, and which will hereafter be called by its true name of proboscis. It happens that bees can easily bend the proboscis downward or toward their own body, but only with considerable difficulty can they bend it in the opposite way. And yet the honey in the curved tip of the columbine can only be reached by curving the proboscis to fit the tube, and in this process the bee’s body for nearly half its length is forced to touch the anthers. While these are close to the stigma, they produce pollen only on their outside surface, where it is, of course, scarcely likely to reach the stigma, but must be brushed off by the contortions of the bee’s body in reaching the honey. The hairy body of the bee, coated with pollen, goes next to perhaps an older flower of the columbine. Heedless of any change in the flower the bee goes straight for the honey in one of the spurs, again catches hold of the only available support in the center of the flower. But this time, instead of brushing pollen off the exposed anthers, it brushes it off its own body to the stigmas, which, at a slightly later stage than in the one just described, are branched and perfectly adapted for collecting the pollen with which the bepowdered bee can hardly avoid dusting them. Cross-fertilization is of course assured, but it seems a precarious business at best, as the number of bees with a proboscis long enough to do the work is limited. The columbine, by a kind of uncanny foresight, is so constructed that bees or other insects that try to reach the honey and are not provided with a sufficiently long proboscis, nevertheless in further attempts upon other flowers, inevitably cross-fertilize them without reaping their reward. One or two kinds of bees, as though in retaliation for this subterfuge of the columbine, make short work of the honey by biting a hole in the spur and forthwith sucking out the honey without so much as touching anther, pollen, or stigma. The reply of the columbine to this ravaging of its chief attraction is that finally, as a last resort, and by a new movement of its reproductive organs, it is self-fertilized. Here the shape of the flower, the original position of the pistil and anthers, the exposure of pollen only in such a direction that, while a chance of cross-fertilization still exists, it can hardly ever fertilize its own stigmas, all point to cross-fertilization as the plant’s greatest requirement. And yet failing this, it falls back on self-fertilization rather than endure barren sterility.
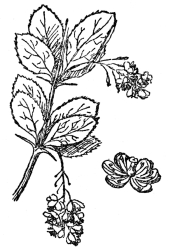 FIG. 70.—COMMON BARBERRY The stamens of this, two at a time, drive off bees by sharp blows, thus preventing self-fertilization.
FIG. 70.—COMMON BARBERRY The stamens of this, two at a time, drive off bees by sharp blows, thus preventing self-fertilization.
While the columbine by its spurs and other interior structure succeeds nearly always in holding a bee long enough to insure its being dusted with pollen, the common barberry bush of Europe (Figure 70), also much planted in American gardens for ornament, actually drives bees away by sharp blows of its stamens, so that self-fertilization shall not result from the visit. In this shrub the petals partly cover the stamens unless the latter are disturbed, and, in fact, the curved tip of the petals forms a kind of socket into which each of the six stamens are fitted. The position of these is such that any insect
Plants of the Palm Family, Palmaceæ, in Ceylon. They are the talipot palm (Corypha umbraculifera), the fiber from the wood of which is used in India for paper making. (After Reinhardt. Courtesy of Brooklyn Botanic Garden.)
A Giant Herb of the Banana Family, Musaceæ. It is the travelers’ tree (Ravenala Madagascariensis), the sheathing leafstalks of which hold considerable quantities of water—hence the name. Grown throughout the tropical world. (Courtesy of Brooklyn Botanic Garden.)
can go straight to the honey glands which are at the base of the flower, without touching the anthers. But their filaments are broadened out at the base, so much so that their edges touch. The honey glands are so placed that the insect must touch the broad bases of at least two filaments, between which, in fact, it must force its proboscis in order to reach the honey. The moment any particular pair of filaments are irritated by the bee, two pollen-dusted stamens fly out from their position among the petals and the anthers strike the bee with a sharp blow. Many observations prove that almost never does the bee go on with his honey sucking after this rude interruption, which has resulted in at least its head being dusted with pollen. The low, sticky stigma is so placed that it is one of the first things the bee’s head strikes as it reaches the center of the flower. Because of the position of the stamens, while they are undisturbed, it is impossible that pollen from them could have been brushed off at the bee’s entrance of the flower. And by an almost miraculous adjustment of the power of the blow by the irritated stamens, this drives off the intruder only after he has brushed his pollen-laden head over the stigma. His head at this stage is, of course, covered only with foreign pollen gathered elsewhere, but just as soon as the bee tries to get what he came for, sometimes even before he gets his reward, out fly the pair of stamens, thoroughly dusting the bee, and seeing to it that the blow is just sufficient to drive off the pollen-laden insect. No device to secure cross-fertilization could be more effective. If the blow of the stamen were only ever such a slight fraction less than it is, the bee would only stop a moment and then go on honey sucking; which, because of the release of the pollen by even the gentlest blow, would result in self-fertilization by the aid of the bee, rather than cross-fertilization. Very few, if any, bees will stand this gentle reminder to go, however, and it is a little curious that such intelligent creatures as they are supposed to be, should not realize that it is all the clever but quite harmless trick of an apparently still more intelligent flower to secure fertilization from any pollen but its own.
To attract insects and then repel them seems a little like using them as some flirts notoriously use men, only to throw them over when they are no longer interesting. In the large-flowered magnolia tree from the southeastern United States, insects, however, fare somewhat better than this. In this magnolia, which has flowers several inches long, self-fertilization is impossible as the stigmas are ready to receive a mate several days before the laggard stamens are provided with the wherewithal. Without some insect or other outside help there would be only a childless old age for this particular tree. The flower opens rather early in the season, while the nights are still cool, and as a protection from the cold, rose beetles habitually fly into them. They find a pleasant shelter under the three inner petals which arch over the honey-coated stigma, and form a snug little chamber so much warmer than the outer air that its heat is appreciable to the touch. The rose beetles, once they are inside this warm shelter, cannot get out and are often held for a few days. Then, as the stigma passes its period and the stamens are furnished with pollen, the chamber opens by the gradual withering of the petals. But the insects, in their efforts to get out, have raised a perfect dust storm of pollen with which they are naturally covered. Just as soon as they are released they are free to seek another warm shelter where the process is repeated. Thus they always enter the flower with foreign pollen, use it up impregnating the waiting ovule, and are held until the flower’s own pollen gives the signal for their release properly dusted for a renewal of the work. The premature timing of the stigma, the tardiness of the anther in producing pollen, the generation of heat and secretion of honey which frequently covers the whole stigma—there could scarcely be a better equipment for securing cross-fertilization. And without it the magnolia would be simply sterile.
There are some other flowers that hold visiting insects in a trap until cross-fertilization has been completed, and all of them by no means furnish their visitors such a snug little heated chamber as the magnolia. One vine from the eastern United States, known as the Dutchman’s-pipe, or sometimes as the pipevine (Figure 71), is singularly ruthless in this respect. Its flowers are of such evil odor that only carrion-loving insects, such as certain kinds of flies and gnats, ever visit them. The flower is of very peculiar structure, being formed of a hollow tube bent from its stalk first downward, and then upward. The upper part ends at the opening which is provided with a three-lobed lip or doorway. Through this the insects crawl, and they finally reach the bottom of the curved part of the flower. Behind and above them is the entrance through which they have just come. And above them, in the other curved part of the flower, is the stigma. As in the magnolia, this matures several days before the pollen from its surrounding stamens is ripe, so that self-fertilization is never possible. What is now the
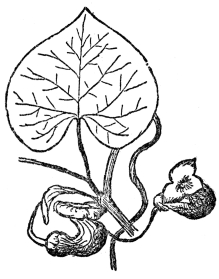 FIG. 71.—THE DUTCHMAN’S-PIPE A vine producing evil-smelling flowers, which trap insects sometimes for days, thus insuring cross-fertilization.
FIG. 71.—THE DUTCHMAN’S-PIPE A vine producing evil-smelling flowers, which trap insects sometimes for days, thus insuring cross-fertilization.
plight of the insect at the bottom of the upward-pointing tubes, one leading to the organs of reproduction, the other to the exit? By an almost diabolical cunning the inside of this flower is so smooth that no insect can crawl up its slippery sides. It takes some time for the prisoner to find this out, and, in the meantime, it has explored every nook and corner of the flower by flying. In the course of this exploration it reaches and covers the stigma with pollen, for as we shall see presently, it always comes into the flower pollen-laden. Evidently becoming panicky about getting out, the insect then flies with very considerable force in every direction. Toward the true exit it naturally flies the most, and by a refinement of cruelty this is the lighter end of the flower, and therefore the obvious mode of escape for it. But the three lips and the entrance they cover are not flat across the flower, as the cover of a lunch box would normally be, but turned at such an angle as the lunch box would be if set on end. The insect, in flying toward the light, invariably hits the smooth surface just inside the three lips and falls to the bottom of the flower because there is nothing rough enough for it to cling to. Throughout most of the day, and sometimes for several days, the insect will keep up this ceaseless struggle to escape, flying first up one tube and then the other. These frantic efforts would end in exhaustion if kept up too long, and before that happens, but after the impregnated ovules have no further use for the flies, the anthers give forth plentiful supplies of pollen. Of course, the insect can scarcely avoid becoming covered with this, and then, but only then, the flower begins to wither and up its now wrinkled sides the pollen-laden prisoner can at last crawl to liberty.
There appears nothing very romantic about the cross-fertilization of the Dutchman’s pipe, in fact, the whole affair seems but a sordid and, it must be confessed, a very efficient trick to get what the flower needs from the insect, rewarding it by many hours of apparently hopeless captivity. But most flowers do have something that insects want, and none so well fulfill the expectations of butterflies as the meadow pink. This is a graceful little perennial native in the fields in central Europe, but often grown in American flower gardens. It has beautiful pink flowers, with a long tubular calyx, at the bottom of which are rich honey glands, accessible only to the long proboscis of different kinds of butterflies. No other insects, and many try, are able to get the honey from this plant. It begins flowering by opening its five beautifully fringed petals, all of which are marked with lines from their edge toward the center. These obvious “pathfinders” are common on many other flowers and all seem to be there to act as a guide for alighting insects, and, as it were, steer them to the center of the flower. As a butterfly alights he finds five protruding anthers covered with pollen. These are of no use to him, but nevertheless his head is covered by their pollen. In fact, many other visitors, who can never reach the honey, find the bright color and good stocks of pollen sufficient attraction for them, and after a pollen feast fly off to other flowers. If the butterfly comes at this state of the flower’s life, it pokes its long proboscis down into the tube of the calyx, but finds the passage almost blocked by another set of five stamens not yet ready to discharge pollen, and, as though ashamed of the fact, quite hidden from view. Also, the proboscis is very nearly stopped by the style, which, if its stigmas were ready for mating, would then and there become impregnated. But they are rolled together into a tight spiral, and their pollen-receptive faces tightly pressed together in addition to the whole structure being twisted, so that the very probably pollen-laden proboscis of the butterfly finally gets to the honey without leaving one grain of pollen where it could possibly self-fertilize the flower. Honey-laden, it now flies away, and so far as this particular flower is concerned, simply nothing has happened but to coat several insects with pollen. A little later the anthers of the first set of stamens cease work and drop off. Then the five stamens hitherto hidden in the calyx burst out and furnish a second crop of pollen, but the stigmas are still safely coiled away from the possible danger of self-impregnation. During this second stage the flower may repeatedly be visited, but until this second crop of anthers become useless there is not the slightest risk of the stigmas becoming self-fertilized. For the hole through which the second set of stamens has come is so small that still only the proboscis of the butterfly can penetrate it. When finally the second crop of anthers also fall off, then the style slowly uncoils and thrusts its now receptive stigmas above the calyx and in plain view of passing visitors. These come, pollen-laden of course, from other flowers, and cross-fertilization is assured. Here the flower furnishes two crops of pollen, plentiful supplies of honey, and asks only that in gathering these the butterflies see to it that its stigma be covered only with foreign pollen. Keeping carefully out of the way while they are about their business and there is danger of self-fertilization, they come out boldly once that danger is past. In this meadow pink self-fertilization is simply impossible; everything in the production of its young it owes to the butterflies, to which it surely makes adequate returns.
While such plants as the meadow pink and thousands of others have lost, if they ever possessed, the power of self-fertilization, and rely absolutely on insect visitors for their perpetuation, there are many hundreds of kinds that apparently hope for cross-fertilization, but, in default of it, due to their inability to absolutely compel it, they finally accept self-fertilization as a last resort. Darwin once said that “Nature abhors perpetual self-fertilization,” and the frequent visits of insects and their rôle in preventing it, together with the flowers’ adaptations to such visits, support the contention, which has never been seriously questioned. But some flowers appear to have left the back door open, as it were, so that failing cross-fertilization, they may still rely upon self-fertilization. A geranium from the Pyrenees, a relative of our common woods geranium, is of this type. A day or two before its stigmas are ripe it produces first one set of five anthers, all pollen-coated, and then another. If these have not been brushed clean, as often or usually happens, then the stigmas ripen and, of course, are impregnated by their pollen. If they are brushed clean of pollen, then the stigmas must rely on foreign pollen, of which it is assured a supply from the visits of pollen-laden insects from other flowers, which are still attracted by the flowers’ color and honey. Perpetuation is assured, in any case, but the preference is still for cross-fertilization.
A more remarkable case of leaving one final chance for self-fertilization is the gas plant. It exhales such a strong and peculiar odor that only certain kinds of insects will visit it. In fact, the odor is so strong and is so heavily charged that a lighted match held near it has been known to slightly ignite—hence the plant’s name. The flower bears a low, squat stigma, profusely covered with honey, which is perfectly accessible to any insect visitor. It has ten stamens, which at first are quite out of the way of insects, two being folded back in each of the five yellow petals. First one stamen begins moving gently from the shelter of its petal, and the anther, pollen-coated, hovers over the stigma, which would inevitably lead to self-fertilization if the stigma were only ready. It never is, and, as though realizing this, the stamen gently moves back out of the way, still, in most cases, retaining some of its pollen. Then another tries and, again, as though realizing the futility of impregnating the unreceptive stigma, it also moves back. So it goes with all the other eight stamens, each of which moves gently out over the stigma, and gently back again, all of them failing to fertilize the laggard stigma. But there has been during all this time a constant procession of insects coming for the honey, and never for a moment have the stamens been off guard, so that each visitor goes off with at least some pollen clinging to it. Finally the stigma, after all the faithful ten are folded back among the petals, comes into its period, and is cross-fertilized by the insects which come from other flowers laden with pollen, as we now know. But if, by one of those accidents of nature, such as bad weather or what not, no insect ever does come, what then is to be the fate of the stigma? Has it staked all only to lose out in the end? It would almost seem that to ignore the steady attentions of the willing ten might make barren sterility a fitting punishment. But, in spite of what has happened, the stamens come to the rescue, if all insects fail, and this time, in a body, they rise up and shed upon the stigma from the half-withered anthers their few remaining pollen grains. There could be no finer example of having an anchor to windward.
One of the largest families of flowering plants is the pea family (Figure 72), with over five thousand members, practically all of which rely on bees for cross-fertilization. In some kinds, where bees occasionally fail them, the flowers wither without self-fertilization and, of course, no seed are then produced. In such a large family of plants there are
 FIG. 72.—THE EVERLASTING OR PERENNIAL PEA A member of the Papilionaceæ or pea family which rely almost entirely on bees for fertilization.
FIG. 72.—THE EVERLASTING OR PERENNIAL PEA A member of the Papilionaceæ or pea family which rely almost entirely on bees for fertilization.
naturally many different adaptations for securing cross-fertilization—some of them of such extreme complexity that they could hardly be included here. All the family have the characteristic pealike flowers familiar enough in the sweet pea, which have already been described and figured on page 44. In all of these stamens and pistils are hidden inside the keel, at least in the early stages of the flower. In some, such as clovers, for instance, the organs emerge from the keel, and after fertilization by insects re-enter their retreat. There are scores of different plans for securing the desired object, but the common alfalfa, with a few other related plants, has the most startling. The flower begins life with its stamens and pistils concealed within the keel, which is apparently impregnable. When a bee alights on the flower and begins work he is welcomed by a small but violent explosion. When the dust of this clears away, and it is actually dusty with pollen, the dazed bee is seen to have fertilized with almost instantaneous rapidity the stigma of the exploding flower, which springs violently out of the burst keel. This is so arranged that, as it flies out of its trap, due to the explosion touched off by the bee, it strikes the under side of the bee’s body a distinct blow, brushing off in the process the pollen nearly always found there. This pollen has come from other plants. But, as in most plants of the pea family, the stamens closely surround the stigma, but are shorter than it. When the explosion occurs, the stigma, because it is slightly longer than the stamens, is the first thing to strike the bee’s body. Already impregnated, it is then indifferent to the cloud of pollen from the stamens of its own flower, which only a fraction of a second later also strikes the bee’s body. The first flower that a bee visits cannot, of course, be cross-fertilized notwithstanding the explosion which results, no matter from what angle the bee attempts to insert its proboscis. But in this case, as the stigma is unfertilized by foreign pollen, its own performs that service. In the vast majority of cases cross-fertilization is assured by certainly the most novel of processes, and, in the rare event of the bee not being covered with foreign pollen, self-fertilization is still possible.
In many of the plants already noted cross-fertilization is accomplished by virtue of the fact that the stamens mature before the stigma. But in the common strawberry the reverse is true. As the insect stands on the white petals, it must, in order to reach the honey at the base of the flower, put its head down so that if pollen were available there could only result self-fertilization. But while the stigma is ready, the pollen at this stage never is, and the insect which comes usually laden with pollen from other flowers cannot avoid impregnating the stigma with this foreign pollen. Later the stamens mature and, as insect visits continue so long as honey is to be found, they become dusted with pollen which is used for the fertilization of other plants. Scores of other plants also produce ripened stigmas before the pollen matures, and they must rely on insects to cross-fertilize them.
The cross-fertilization of the strawberry is such a comparatively simple process—seems in fact almost inevitable—that we are lost in wonder at the almost mathematical complexity of the act in the common purple loosestrife, which has been introduced into American gardens from Europe, and sometimes runs wild. In this plant there is a long terminal spike of showy, purplish-pink flowers, the color of which is sufficient to attract many insects from even a fairly swift flight. The petals are streaked with “pathfinders” toward the center of the flower. This consists of a tubular calyx; at the bottom of this is the honey, which secures the insect’s further interest once the color has attracted it. But it finds a condition of the reproductive organs almost without parallel. In some plants the style is hidden down in the calyx tube, while one set of stamens just peep out of the end of the tube, and a second set are still further and quite obviously protruded. In a neighboring plant the style will be found outside the tube, one set of stamens hidden in it, and the other set outtopping everything, except the petals. In still a third type of the loosestrife, the style exceeds everything but the petals, one set of stamens just emerge from the tube and the lowest set are hidden. It would appear as if the loosestrife could scarcely escape self-fertilization, except possibly in that form where the stigma outtops all the stamens, and this would result always if the pollen were indiscriminately useful from all three lengths of stamens. But it never is, only that from the short-stamened plants will fertilize the short-styled one, the mid-stamened ones the mid-styled counterpart, and the long-stamened ones the long-styled flower. The pollen grains of the different-lengthed stamens are even of different size and color. If this were not so, it is a simple mathematical problem that, with three different sets of style lengths and six sets of stamens, two in each flower, eighteen different crosses might be possible. As it is, only six crosses are ever possible and these only by the aid of insects, for it must be remembered that stamens and pistils of the same length are never found in the same flower. By an adjustment of the size of the body of the different insect visitors it works out so that, while all three body sizes frequently visit each flower, only that particular size of insect suited to the carrying of pollen from stamens of one definite length to a style of similar length actually accomplishes the cross-fertilization of that particular flower. And this without interfering with the visit of another different-sized insect which will accomplish the work for its particular set of stamens and pistils. So marvelous is the adjustment of style length and stamen length to each particular body size of the visiting insects, so perfect is the arrangement of the organs in each flower, that each contributes to the fertilization of its neighbors, never to its own, nor does it interfere with the process in other lengthened styles. Cross-fertilization is thus almost always accomplished in the six different combinations possible in this truly remarkable case of adaptation between insects and flowers.
From the almost mathematical complications of the cross-fertilization of the loosestrife it seems a far cry indeed to that of the Italian honeysuckle. This often runs wild over fences, but is unlike the more widely known Japanese honeysuckle, in that its stem passes through the different pairs of opposite, bluish-green leaves, which are joined together at the base. The Italian honeysuckle falls back on the more simple seductions of odor and honey for securing its really important insect visitors. It has such a long tube that only certain night-or evening-flying moths or butterflies can reach the honey. There is, even during the day, such a plentiful supply of this that it frequently fills half the tube, but even then it is quite out of reach of bees which never succeed in getting any. Toward evening, particularly on quiet, still evenings, the flower begins to send off in much increased quantity a heavy rich-scented odor almost overpowering in its sweetness. The butterflies and moths of the dusk having a long proboscis, succumb to this really enchanting lure, which, with the large store of honey, insures quantities of eager suitors. The stigma, while ripening simultaneously with the anthers, protrudes beyond them, so that the butterflies touch pollen only after touching the stigma, which of course is impregnated with pollen from an earlier visit of the insect to a different flower. So assiduous are the butterflies, that on a still night there will be not one grain of pollen left on the much-brushed anthers. If the night is cold or windy much pollen remains and cross-fertilization is left to pollen-eating insects such as mother bees or flies. While these can never get the honey they often do accomplish cross-fertilization and, sometimes by misadventure, self-pollination. It is only those moths and butterflies which, forcing their long proboscis down the honey-laden tube, must accomplish cross-fertilization. But failing this, the plant is more or less at the mercy of hosts of insect triflers, mere pollen eaters, who may or may not insure cross-fertilization, but in any case provide for self-fertilization.
Another use which certain plants make of honey, besides acting as a lure of insects, is found in the common lilac. The flower in this has considerable honey at the bottom of the tube, which can only be reached by insects with a proboscis sufficiently long to reach it. The lilac has only two stamens inserted near the top of the tube, the passage to which they very nearly obstruct. The stigma is hidden in the tube, and it matures simultaneously with the ripening of the pollen. As the insect inserts its proboscis between the stamens no pollen clings to it due to the character of the pollen grains. But as the proboscis is withdrawn from the tube its lower end is covered with honey to which pollen sticks. If a needle is inserted between the stamens and pushed only far enough to be still clear of the honey, no pollen will be found on it when withdrawn, but if pushed all the way down, its honey-coated point will catch considerable pollen. In the lilac, if insect visitors do not accomplish the work of cross-fertilization, the flower is self-fertilized ultimately by the protruding of the stigma far enough out of the tube to catch some of the remaining pollen grains.
It is perhaps useless to multiply instances of flowers which by various devices secure the cooperation of insects in getting pollen from a foreign source. To recapitulate some of those devices it is necessary only to recall what some flowers have done to force cross-fertilization. The heated chamber of the magnolia, the cruelty of the prison cell in Dutchman’s-pipe, the blow from the stamen of the barberry, the faithful rotation of the ten stamens of the gas plant, the explosive flower in some members of the pea family, the lure of honey and seductive odor of the Italian honeysuckle, the mathematical complexity of the loosestrife—these and hundreds of others all point to the necessity of cross-fertilization and a means to produce it almost beyond belief. There is the best of evidence that not only flowers but insects themselves have been modified in this great work, and that for every flower needing cross-fertilization some agency has been developed to secure it. Insects, beyond all other animal life, do this work, but it is accomplished by humming birds often, and in one plant even by a snail.
Two of the very largest plant families, not so far mentioned in this account, depend almost absolutely upon insects. In the daisy family, with over eleven thousand members, the large heads of flowers, often containing scores of individual flowers, are constantly brushed, over and over again, by the pollen-coated bodies of insect visitors. And in all or nearly all orchids (Figures 73-75), comprising over five thousand kinds, the same process is accomplished. In these plants, in fact, the act is, if possible, more
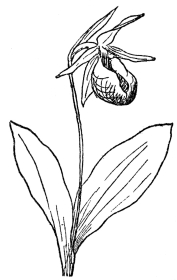 FIG. 73.—PINK LADY’S SLIPPER (Cypripedium acaule) A native orchid in northeastern North America.
FIG. 73.—PINK LADY’S SLIPPER (Cypripedium acaule) A native orchid in northeastern North America.
complicated than in any so far noted. Darwin’s book, “On the Various Contrivances by Which British and Foreign Orchids Are Fertilized by Insects,” reads like a fairy tale. Yet it is the result of years of patient observation by incomparably the greatest naturalist of recent times. To it the reader must go for the details of a drama of absorbing interest, but too long to sketch even briefly here. Perhaps one illustration may be mentioned of how far the principle of cross-fertilization has been carried, and to the deadly effects of its failure in at least one case. In a certain orchid from Brazil, known as butterfly orchid, the pollen is nearly always carried out of the flower by an insect visitor, but, if by mischance it is not, and falls on the stigma, not only does it fail to fertilize the ovules, but it kills the pistil forthwith. There may be a few other cases of such drastic results of self-fertilization, but in any case, and disregarding these apparently suicidal fanatics, cross-fertilization is so very nearly universal that nature must find it of enormous advantage. Only in this way is it possible to explain the intricate adjustments of insects and flowers, which work together in such wonderful harmony that cross-fertilization hardly ever fails in those flowers where it appears to be necessary.
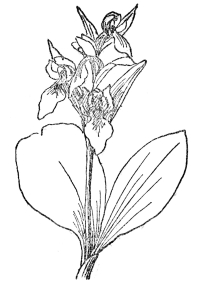 FIG. 74.—SHOWY ORCHID (Orchis spectabilis) Native of eastern North America, with showy magenta-pink or white flowers in a loose raceme.
FIG. 74.—SHOWY ORCHID (Orchis spectabilis) Native of eastern North America, with showy magenta-pink or white flowers in a loose raceme.
It must have struck many thoughtful readers to ask a rather obvious question at this point. Why, if untold millions of insects are constantly flitting from flower to flower, does not the pollen get mixed, as it is quite certain that they will not fly from a
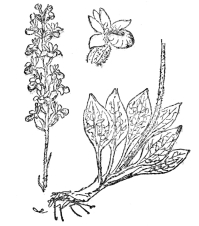 FIG. 75.—RATTLESNAKE PLANTAIN (Peramium pubescens) One of the few orchids native in eastern North America, with white variegated leaves. It grows in dense patches and bears free-blooming spikes of whitish flowers.
FIG. 75.—RATTLESNAKE PLANTAIN (Peramium pubescens) One of the few orchids native in eastern North America, with white variegated leaves. It grows in dense patches and bears free-blooming spikes of whitish flowers.
certain kind of geranium to another similar one for instance, but perhaps to a rose? The answer to this is simple enough, but its implications are limitless. Only pollen of a certain species or variety is useful to the stigmas of that variety. To practically all others the stigma is simply unreceptive, except in those closely related plants that may all have a common parentage. When crosses between such closely related plants do occur the result is known as a hybrid, which will be considered elsewhere. To this extent, then, flowers are peculiarly exclusive in their matings and promiscuity occurs in the vast number of cases only in plants of the same species. We do not yet understand the impotence of pollen of one species upon another; all that we do know, which has many times been proved by experiments, is that it fails to act. If it did act, no one could picture the chaos into which the vegetative world would be thrown.
WIND AND WHAT IT DOES FOR FLOWERS
While, as we have seen, thousands of plants rely upon insects for producing their young, still other thousands put everything to the hazard of the wind. Pollen is so light that it can easily be blown very great distances, and while the wastage is enormous, the process works so well that the greater part of the vegetation of the earth is thus fertilized. This is true not as to the number of different kinds of plants, for in that respect insect fertilization is more important than that accomplished by the wind. But in the number of individual plants concerned the wind is incomparably the greatest fertilizing agency that is known to us. This for the reason that all grasses and sedges, most catkin-bearing trees such as oak, hickory, birch, practically all pine trees and their relatives rely wholly on the wind for fertilization.
For reasons that will be enlarged upon in another chapter, all of these great groups of plants must be considered as of simple structure, some, like the pines, relics of a remote past when no flowering plants, as we know them to-day, existed on the earth. In any event the reliance upon the wind is certainly hazardous, and while it of course insures nearly universal cross-fertilization, it may well result in scanty fertilization or, in exceptional cases, complete failure of it. Quite obvious also is the amount and direction of the wind in the process, for in very open and windy places grasslike vegetation, or at least a predominance of species fertilized by wind, is likely to be found, rather than those plants that rely upon insects, that, unable to stand the full force of the wind, seek more sheltered places. While such a thing is not the cause of prairies, or the predominantly grasslike vegetation along sand dunes, or the exclusive spruce forests of the bleak and windy north country, it unquestionably aids in maintaining the often exclusive nature of such pure associations of plants. Over thousands of square miles on our own great plains or on the steppes of Russia, both subject to violent winds, the great bulk of the vegetation is wind fertilized. It could hardly be expected that pollen, once in the grip of such a wayward and shifting thing as the wind, should not be wasted in great quantities. This is particularly true of pine trees, which at pollen time may often be seen giving off golden clouds of dust, of which perhaps 95 per cent is wasted.
WATER AS AN AID TO FERTILIZATION
Those submerged aquatic plants upon which neither the winds nor honey-seeking insects can work the magic of cross-fertilization, seem to be about the poorest equipped for perpetuating their kind through impregnation of their tiny flowers. And yet, for at least two of them, which will be described presently, the process is accomplished by an adaptation of their mode of life to their watery environment that seems incredible. These two have been selected as illustrating two peculiar adaptations in the weight of pollen or pollen-holding flowers that is common to some other submerged aquatic plants. In one the male flower, or pollen from it, with the very nicest adjustment of function to environment in all the realm of the plant world, is just of the right specific gravity to float to the surface with dramatic suddenness and perfectly timed effectiveness. In the other the pollen is just enough heavier than the water to float betwixt the surface and the bottom, so that at the proper moment it is where it can fulfill its destiny.
The common eelgrass or tapegrass is a submerged aquatic which roots in the mud and has long grasslike leaves which may often be seen waving gently in the current of many quiet streams in this country and in Europe. Down near the base and in among its swaying verdure, it bears tiny flowers which have no petals, and in which, as if recognizing the futility of display in such a secluded watery home, even its calyx is reduced to small scales. Some of these minute flowers are females, others again all males, and as they appear in their early stages it looks as though never the twain could meet. And the hopelessness of their ever meeting is increased as the maturing female begins slowly to uncoil the fine stalk upon which it grows. Steadily but surely the loose spirals of the stalk of this ever more mature female flower uncoils, until, when quite ready for the pollen, it is at last upon the surface. The male flowers, in the meanwhile, are down near the bottom with their small freight of pollen ready to perform their function, but firmly anchored to a stalk absurdly inadequate to reach the surface where alone they can be of service. A great Belgian, Maurice Maeterlinck, who studied this plant with more sympathetic vision than any botanist has yet been able to equal, wrote in one of his essays on “The Intelligence of Flowers” the solution of this little drama of apparent hopelessness. No other words can ever convey the meaning of what happens to the eelgrass quite so well as his. “Is there any more cruel inadvertence or ordeal in nature? Picture the tragedy of that longing, the inaccessible so nearly attained, the transparent fatality, the impossible with not a visible obstacle! It would be insoluble, like our own tragedy upon this earth, were it not that an unexpected element is mingled with it. Did the males foresee the disillusion to which they would be subjected? One thing is certain: that they have locked up in their hearts a bubble of air, even as we lock up in our souls a thought of desperate deliverance. It is as though they hesitated for a moment; then, with a magnificent effort, the finest, the most supernatural, that I know of in all the pageantry of the insects and the flowers, in order to rise to happiness, they deliberately break the bond that attaches them to life. They tear themselves from their peduncle, and, with an incomparable flight * * * dart up and break the surface of the water. Wounded to death, but radiant and free, they float for a moment beside their heedless brides and the union is accomplished, whereupon the victims drift away to perish, while the wife, already a mother, closes her calyx, in which lives their last breath, rolls up her spiral, and descends to the depths, there to ripen the fruit of the heroic kiss.”
In the eelgrass it is the specific gravity of the male flower, or, the secreted air bubble, which makes the flower lighter than the water, and actually causes the flight from the depths to the surface. Because of this, fertilization can only take place on the surface, although the flowers and fruits otherwise mature under water. But in sea wrack, in Naias, and in ditch grass, all submerged aquatics, the flowers are even fertilized under the water. Pollen in such plants is much modified, and instead of being in the ordinary form of pollen grains, it is, at the time of ripening, lengthened out into tubular, hairlike structures. These delicate prolongations of the male fertilizing stuff are carried by the currents of the water, just as a thread would be, but with the difference that the pollen threads are so beautifully weighted to fit their watery environment that they float, suspended, in the depths of the water at or near the level of the female flowers. The pollen is set free at maturity, just as it is in the eelgrass, but to meet the female, which never rises, it must float with the current of the stream. There must, as in the wind-carried pollen, be a tremendous wastage, yet sufficient quantities of it do fertilize the females, particularly in the ditch grass, which fruits very freely.
Whether it be any of the various contrivances for insect fertilization, or by the winds, or, as in the eelgrass, by the water, the climax of the flower’s life is always reached in this act. For all annuals the plants, also, begin to die down then, a process that is completed with the production of seed, which is, of course, the object of all those varied modes of fertilization. Perhaps no answer to the question of why plants do not always self-fertilize themselves is so eloquent as the hundreds of ways they have adopted to avoid doing so, a few of which we already know. Many volumes have been written on this subject, but all of them, intricate as the methods they describe nearly always are, merely confirm what we have already seen—that rather than submit to self-fertilization, plants will adopt almost undreamed-of expedients. Sometimes, as in the eelgrass and in the visits of nocturnal insects to those night-blooming flowers that carry on their matings in the glamour of moonlight or in the dusk of eventide, the drama, in the eyes of imaginative writers, is one of singular beauty and charm. And, on the other hand, we have seen the well-nigh heartless cruelty of the Dutchman’s-pipe in keeping as prisoners its absolutely necessary insect deliverers. Even this is outranked for matchless ruthlessness by a wild arum, a relative of our jack-in-the-pulpit, from the East Indies. It produces a club-shaped inflorescence composed of tiny flowers that need cross-fertilization, but so offensive is the odor of the flower that no insects will tolerate it. A snail, a voracious eater of foliage, is attracted to the flower partly by the fine fleshy leaves, but mostly by a juice secreted at the apex of the flower column. To this the snail crawls, and fertilizes the tiny flowers over which it drags its body. When this is accomplished it speeds on hungrily to the juice just above it and eagerly devours the poison. Death follows almost immediately. The secretion of this murderous liquid to lure the only creature that will visit such an offensively malodorous plant, which, without it, would very likely be itself destroyed by the foliage-eating snails, is a gruesome contrast to that happy flitting of butterflies which completes the fertilization of most flowers in equally effective but more pleasing fashion.
Once impregnation of the ovule has been consummated, it begins a slow process of change, involving sometimes the modification of the ovary, or of the calyx, and very often of the swollen apex of the flower stalk upon which these organs are borne, known technically as the receptacle. We have seen, in the first chapter, what greatly different types of fruits are developed from different ovaries, and they of course produce seeds in varying size and amount. In the coco de mer, a palm from the Seychelles, the seed often weighs forty or fifty pounds, while in some orchids a single capsule will contain over a million almost microscopic seeds. Some of the devices of fruits and of seeds to secure the utmost spreading of the species over the earth will be considered in another chapter. All the devious methods of plants in producing their young become significant, so far as the earth’s vegetation is concerned, only when we find out what this enormous progeny has done with their opportunity. The chapter on the Distribution of Plants will tell us how well that opportunity has been used.